
Identifying your child’s mutation.
EXONDYS 51 is used to treat Duchenne muscular dystrophy in patients who have a confirmed mutation in the dystrophin gene that can be treated by skipping exon 51.
EXONDYS 51 was approved under accelerated approval. Accelerated approval allows for drugs to be approved based on a marker that is considered reasonably likely to predict a clinical benefit. EXONDYS 51 treatment increased the marker, dystrophin, in skeletal muscle in some patients. Verification of a clinical benefit may be needed for EXONDYS 51 to continue to be approved.
Meet Liam, age 19
Deletion of exons 48-50
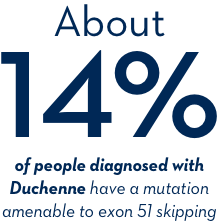
What does amenable mean?
Amenability describes a potential responsiveness to treatment. If your child’s genetic mutation is a deletion of one or more exons in the dystrophin gene that are amenable to exon 51 skipping, then it may be responsive—or, amenable—to EXONDYS 51.
If your child has been diagnosed with Duchenne, ask your doctor about genetic testing.
The goal of a genetic test is to identify the genetic mutation so treatment options can be considered. To learn if your child’s mutation is amenable to treatment with EXONDYS 51, take the immediate step of asking your doctor to order a genetic test.
Understanding your child's mutation and amenability.
Your doctor or genetic counselor will be able to interpret the genetic test results to identify the genetic mutation and determine amenability to treat with EXONDYS 51.

Reexamine or retest.
If you’ve had a genetic test, it’s important to know that testing methods have changed over time and may now provide more accurate or additional information. Ask your doctor to review older test results or request a new test so they can determine if your child’s deletion is amenable to exon 51 skipping.
Ask your doctor if a genetic mutation was identified in your child’s genetic test.
What can I learn from a genetic test?
A genetic test will:
- Confirm a diagnosis of Duchenne so you can take action on your child’s behalf
- Identify the genetic mutation so treatment options can be considered
- Assist with family planning
We know the mutation. Is my child amenable?
EXONDYS 51 is intended for patients with Duchenne who have genetic mutations that are large deletions—where one or more specific exons, listed below, are missing. A doctor or genetic counselor will need to interpret your child's genetic test results to determine if your child can take EXONDYS 51. If the genetic mutation is not amenable to treatment with EXONDYS 51, talk to your doctor about other potential treatments.
The deletions, or ranges of deletions, that are theoretically amenable to exon 51 skipping are shown here.
Choose an exon range.
Find out how EXONDYS 51 may help the body make a shorter form of the dystrophin protein.
This educational table is not intended to make a diagnosis or replace discussion with your doctor or genetic counselor.
*Patients with DMD who have a deletion of exon 52 may be treated with a therapy that skips either exon 51 or exon 53.


Meet Xavier, age 10
Deletion of exons 45-50
If your child has been diagnosed with Duchenne, talk to your doctor to see if EXONDYS 51 is right for your child.
“Families receive this diagnosis and sometimes they don’t know what’s next. SareptAssist Case Managers are here to help families navigate the process of starting treatment with EXONDYS 51.”
-Ashley, SareptAssist Case Manager

Related FAQs
A mutation is a change in a person's DNA. Mutations range in size from a small (a single rung on a ladder) to a large segment of DNA. Every mutation causes a different effect on our bodies. Learn more about the role of genetics in Duchenne.
A deletion is a type of mutation where genetic material is missing. Duchenne is caused by a mutation on the dystrophin gene; the mutation may be a deletion, duplication, or other change in the gene. Learn about genetic testing for Duchenne.
Please discuss your child's genetic test results and treatment options with your child's doctor. For EXONDYS 51 to work, exon 51 must be present in the dystrophin gene. Your child's doctor or a genetic counselor is the best person to help you understand genetic testing. Explore what genetic test results can tell you.

