What to expect on infusion day.
Get ready for treatment.
Because EXONDYS 51 is given through a weekly intravenous (IV) infusion, you may have some questions about what you can expect and how you should prepare.
Questions?
Give us a call.

1-888-SAREPTA
1-888-727-3782
Case Managers are available Monday – Friday
8:30am – 6:30pm ET
![]()
How long does an infusion take?
The infusion itself will usually take about 35 to 60 minutes. Plan on some extra time for preparing the IV and medicine. If your child experiences any side effects from the medicine, your doctor or nurse may need to slow or stop the infusion.
![]()
We’re going to an infusion center—how should I plan for that?
Usually, you'll need to plan time for check-in, starting the IV, and preparing and infusing the medicine. It’s a good idea to save the travel instructions to your phone or print them out in advance.
![]()
How do I get ready for a home infusion?
Check with the home infusion nurse for information about how to get ready for your child's infusion.
Ask your healthcare provider if there are any other considerations you should be aware of.

Infusion day tips. Keep your child’s favorite comfort item close by and be sure they have a book, movie, or game to help the time pass more quickly. If you’re traveling to your infusion location, allow extra time for traveling, finding a parking spot, and getting your child inside. Arriving on time will make for a more comfortable infusion day—for everyone.
At-home infusions with Ash
Every patient has unique needs and routines. For Ash, being able to have his infusions at home—with his own schedule in mind—was a relief. Watch to learn more about his experience.
Infusion step-by-step.
Here’s what you can expect when it’s time for your child’s infusion.

The nurse may take your child’s vital signs: pulse, temperature, and blood pressure.

The nurse will prepare the access site and may apply a local anesthetic. If using an IV, the nurse will apply a tourniquet and insert the IV through a needle. If using a port, the nurse will use a needle to access the port.
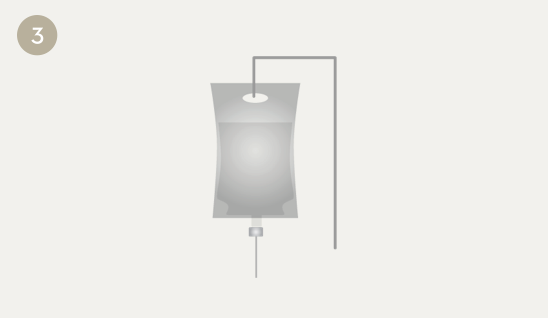
The medicine is added to the infusion bag, a 0.2 micron in-line filter is added to the IV tubing, and the infusion is started.
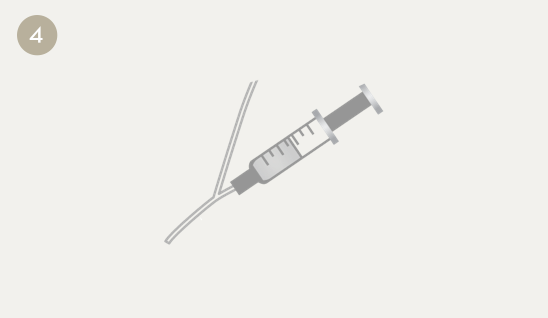
When the infusion is complete (usually after 35–60 minutes), the tubing is flushed with saline to ensure that all medication is received.
“The advice I would give others about infusion is to take your time to think about what the best solution is for you.”
–Ash, EXONDYS 51 patient
Your doctor will discuss the options that might be right for you.

Related FAQs
Talk to your healthcare provider. If you miss a dose of EXONDYS 51, it may be administered as soon as possible after the scheduled time.

