Weekly infusions of EXONDYS 51 (eteplirsen) have been shown to help the body make a shorter form of the dystrophin protein in some boys.

Understanding EXONDYS 51.
EXONDYS 51 is a treatment for Duchenne muscular dystrophy. It uses a technology called exon skipping to help the body make a shorter form of the dystrophin protein. EXONDYS 51 is given in a once-weekly infusion.
EXONDYS 51 is used to treat Duchenne muscular dystrophy (DMD) in patients who have a confirmed mutation in the dystrophin gene that can be treated by skipping exon 51. EXONDYS 51 was approved under accelerated approval. Accelerated approval allows for drugs to be approved based on a marker that is considered reasonably likely to predict a clinical benefit. EXONDYS 51 treatment increased the marker, dystrophin, in skeletal muscle in some patients. Verification of a clinical benefit may be needed for EXONDYS 51 to continue to be approved.
Results from EXONDYS 51 clinical studies.
Researchers conducted clinical trials of EXONDYS 51 to review different aspects of the medicine, including whether it triggers skipping of exon 51 on the dystrophin gene. They also analyzed the amount of dystrophin produced, along with its safety profile. EXONDYS 51 was studied in boys who had a confirmed mutation in the dystrophin gene that could be treated by skipping exon 51. Learn what this means.
Designed to skip exon 51.
Three clinical studies looked at whether exon skipping occurred on the dystrophin gene of boys treated with EXONDYS 51. In clinical studies, exon skipping occurred in all 36 evaluated study participants.
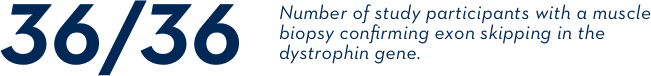

Dystrophin levels increased in some clinical trial participants.
A study of 12 boys with Duchenne muscular dystrophy compared the level of dystrophin in their bodies before the first infusion of EXONDYS 51 with the level of dystrophin after 48 weeks of treatment with EXONDYS 51. Weekly infusions of EXONDYS 51 helped the body make a shorter form of the dystrophin protein.
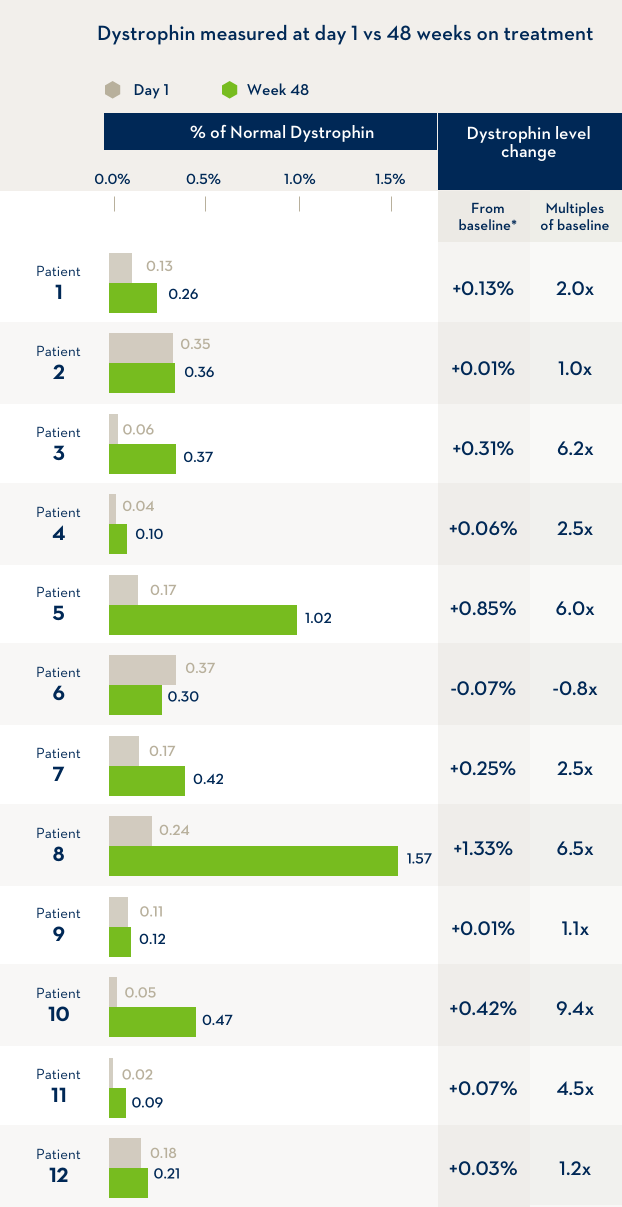
*After 48 weeks, dystrophin levels were an average of 2.8x higher than the level before treatment began, but still remained at a level much lower than that produced by people without Duchenne. In another study of boys treated for 180 weeks, dystrophin levels were an average of 0.93% of the level produced by people without Duchenne. The change in dystrophin levels is the % of normal dystrophin at Week 48 minus the % of normal dystrophin at the start of the study (baseline).

What do the results tell us?
Baseline dystrophin level was
0.16%
of normal
After 48 weeks the average dystrophin level was
0.44%
of normal
but levels still remained much lower than the levels of people without Duchenne
Participants showed an average dystrophin increase of
2.8x
the baseline level
but levels still remained much lower than the levels of people without Duchenne

What about risks? Researchers assessed the risks and tolerability of EXONDYS 51. As with any medication, you should discuss the risks and side effects associated with EXONDYS 51 with your doctor.
Additional study results.
In a separate study of 12 patients, the average dystrophin level for participants after 180 weeks** was
0.93%
of normal
but levels still remained much lower than the levels of people without Duchenne
**Baseline levels were not available for all participants.

