Side effects were evaluated in clinical studies.
As with any medication, it’s important to talk to your doctor about the possibility of side effects with EXONDYS 51. Here’s what you should know about side effects experienced by those receiving EXONDYS 51 in clinical studies.
Allergic reactions can happen.
Allergic reactions have occurred in patients treated with EXONDYS 51, including:
- Wheezing
- Chest pain
- Cough
- Rapid heart rate
- Hives
Seek immediate medical care if signs and symptoms of allergic reactions happen.
Common side effects.
The side effects that occurred at least 25% more often in 8 patients treated with EXONDYS 51 by intravenous infusion than in 4 patients who received a placebo were:
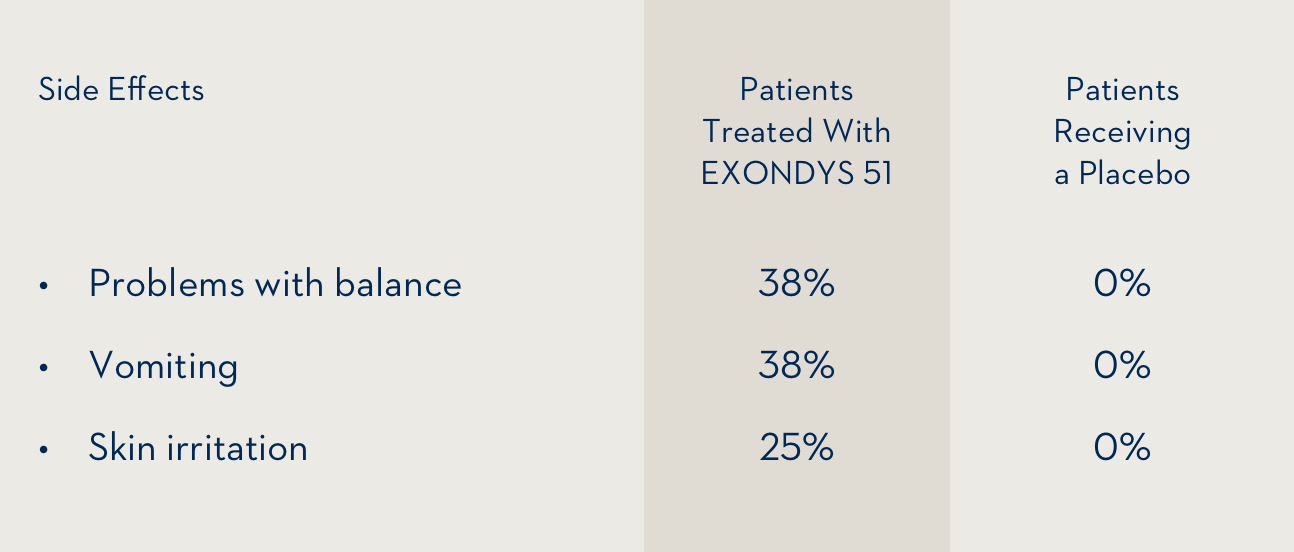
The most common side effects were problems with balance and vomiting.
Additional side effects with EXONDYS 51 seen in other clinical trials.
The following side effects were reported in greater than 10% of patients who received EXONDYS 51 (N=163):
- Headache
- Cough
- Rash
- Vomiting
Seek immediate medical care if signs and symptoms of allergic reactions happen.

A phone call away
As with any medication, please talk to your doctor if you experience any side effects from EXONDYS 51. We encourage you to report negative side effects of all prescription drugs to the FDA. Visit www.fda.gov/medwatch or call 1-800-FDA-1088. You may also report side effects to Sarepta Therapeutics at 1-888-SAREPTA (1-888-727-3782).
Please see the full Prescribing Information for EXONDYS 51 (eteplirsen).
Related FAQs
Weekly infusions of EXONDYS 51 helped the body make a shorter form of the dystrophin protein in some boys. View the results from clinical studies.
EXONDYS 51 is used to treat Duchenne in patients who have a confirmed mutation in the dystrophin gene that can be treated by skipping exon 51. EXONDYS 51 was approved under accelerated approval. Accelerated approval allows for drugs to be approved based on a marker that is considered reasonably likely to predict a clinical benefit. EXONDYS 51 treatment increased the marker, dystrophin, in skeletal muscle in some patients. Verification of a clinical benefit may be needed for EXONDYS 51 to continue to be approved.
